At the end of April, I received an end-stage diagnosis for my throat cancer. What this means is that although there’s no certainty about when I will die, neither I nor my physicians see a likely path leading to a cure. That doesn’t mean that there is no hope. We are working on a new plan of treatment with immunotherapy. In this update, I will explain what immunotherapy does.
My cancer is an oropharyngeal squamous cell carcinoma (OSCC).
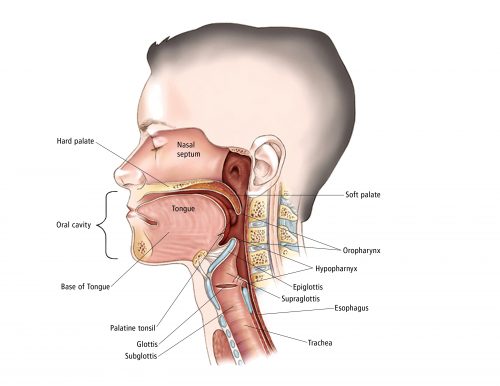
The oropharynx is the region of the throat between the tongue and the epiglottis. In OSCC, the cells that line my throat in that region have started growing in an uncontrolled manner, forming a tumour.
The first line of treatment for OSCC is radiation, and it is usually curative. But unluckily for me, my tumour survived radiation and quickly recurred. It’s now a hard lump, visible on the right side of my neck, with an ulcer inside my throat. Why is a lump in my throat a problem? In order of increasing severity, here is what the tumour does.
First, I feel disfigured by the visible tumour. There is also pain, from the ulcer and because the tumour impinges on the nerves in my throat. The tumour is getting large enough to hinder my swallowing, which is already difficult because of the pain. I may have to go back to feeding through a tube. If the growth is unchecked, it will block my airway, although we can ‘fix’ that with a tracheotomy. Worst of all, a large tumour includes millions of cancer cells, and the more cells there are, the more likely it is that one or more will escape and seed metastatic tumours elsewhere in my body. These, as they grow, will shut down other body systems. This last process is what is most likely to kill me.
In short, if possible, we want to get this thing out of me!
Which is, of course, what cancer treatment is about. In essence, cancer can be treated with radiation, surgery, or medications. The 35 sessions of radiation I received last summer left my tumour unimpressed. More radiation would likely hurt me more than the tumour.
I could have throat surgery, and if the surgeon completely removed the tumour and its local metastases, that would likely cure me.
Unfortunately, my tumour is large and located at the centre of the base of my tongue. This means that the surgery would damage a great deal of tissue. I would likely lose my tongue and much of my larynx, so I would be unable to swallow or speak. The surgery would also damage my epiglottis, the flap of tissue that allows us to switch the throat from a tube delivering air to the lungs to one delivering food and water to the gut. If my epiglottis isn’t working, my lungs will get infected, leading to pneumonia. I would spend much of my post-surgery time in the hospital and I would likely die there.
Think about that: would you want to have to die in a hospital during COVID, possibly alone, and unable to talk to anyone about what is happening to you? Two surgeons have now walked me through the consequences of surgery. Neither hid his relief when I declined the procedure.
This leaves medication. Classical chemotherapies work by damaging the genes in the nuclei of cancer cells. The problem is that they hurt the rest of the body, too, just not as much. But recently, medical oncologists have been developing immunotherapies. The current plan is for me to start treatment with a drug called pembrolizumab.*
To explain how this drug works, let’s take a step back and ask why cancer happens. Cancer happens because something damages the machinery in a cell that regulates the cell’s reproduction, leading it to grow uncontrollably. But that’s not enough to produce a tumour. The tumour also needs to escape the body’s immune system.
The immune system’s T-cells fight microbial pathogens, and they also search for and destroy cancer cells. The T-cells are like the police. If you are a cell, so long as you don’t reproduce more than you are supposed to, then no one gets hurt.
Which is great, except that having armed patrols roaming the body poses its own danger. If the T-cells get too aggressive and start attacking normal body tissues, we have an autoimmune disease. To prevent this, the body has evolved mechanisms that can suppress the T-cells.
One of these is called the PD-1/PD-L1 pathway. PD-1 is a receptor protein on the surface of a T-cell for programmed cell death. It is an off-switch on the T-cell. If another cell can bind to this receptor using a protein called PD-L1, the T-cell kills itself.
But this, unfortunately, provides an opportunity that the tumour can exploit. If the tumour gets a mutation that allows it to express a lot of PD-L1, it can escape the T-cell police and continue its uncontrolled reproduction.
Here is where pembrolizumab comes in. The molecule prevents the tumour from using the PD-1/PD-L1 pathway to shut the T-cells down. If the drug can do this, the T-cells will attack the tumour, which sometimes just ‘melts away.’ Some people have reactions to the drug, including inflammations that might be caused by overactive T-cells. Fortunately, these side effects are typically much less severe than those of older chemotherapies.
Sounds great, no? Well… here are the results from the KEYNOTE-048 trial, comparing pembrolizumab against the previous standard of care. The patients have recurrent head and neck cancer, like me. The horizontal axis is the time since the initiation of treatment. The vertical axis is the proportion of patients who are still alive at that time.
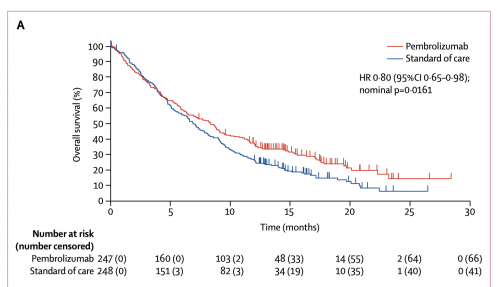
Notice that half of the pembrolizumab patients are dead by 9 months. Pembrolizumab does better relative to standard care. But in absolute terms, the treatment doesn’t accomplish much.
Why doesn’t immunotherapy work better? My cute story above oversimplified the immune system. The system includes a zillion other mechanisms, an alphabet soup of pathways, each seemingly there to correct the malfunction of some other pathway. Here is an animation that illustrates how the immune system functions.
The immune system is a reductio argument against intelligent design: if it had a designer it must have been a committee of lunatic clockmakers, who spoke no common language. Immunotherapy doesn’t work that well because we don’t know what we are doing. You could not pay me enough to be an immunologist.
We are in an odd historical moment in oncology. On the one hand, we have enough data that I can foresee my life expectancy with some confidence. Twenty years ago, I couldn’t. On the other hand, we can’t reliably cure this disease. I hope my great-grandchildren will be shocked to learn that people used to die from cancer.
What matters, however, is the survival curve. Even with my best treatment option, I don’t have much time. So be it. That time is where the hope is. I need to think hard and make good choices about what to do with it.
*Where do these crazy drug names come from? There is actually a system to it (h/t, David States).

