The following is part of a series of guest posts on hepatitis C by Allan Joseph, a medical student at the Warren Alpert Medical School of Brown University and TIE research assistant. You can follow Allan on Twitter: @allanmjoseph. Links to all posts in the series to which this post belongs are in the introductory post. That post includes a glossary of terms as well.
There’s a good review of the evidence on chronic hepatitis C (HCV) treatments in this brand-new California Technology Assessment Forum (CTAF) document, which I’ll refer to throughout and includes most of the figures I’ll use in this series. CTAF produced an impressive meta-analysis of various treatment options — rates for Sustained Virologic Responses and expected drug costs come from that document — which I also used to calculate numbers needed to treat (NNTs.)
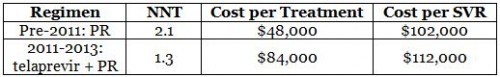
Pre-2011 treatment: the PR regimen
Prior to 2011, the standard of care for chronic HCV was a two-drug approach: pegylated interferon (sometimes referred to as peginterferon) and ribavirin, or “PR” for short. This was a broadly unsatisfactory treatment regimen, mostly due to the side effects of peginterferon.
Peginterferon is a modified version of a molecule your body makes itself when it senses a viral infection. It basically supercharges your cells to fight off the virus. In fact, you make it in high quantities when you get something like, say, the flu. It’s interferon-alpha, your body’s native version, that gives you the characteristic symptoms of the flu: fatigue, headache, and muscle aches.
Peginterferon is given with a weekly injection for 48 weeks. Trying to treat patients who have HCV without symptoms is difficult, because they feel just fine and would much rather not feel like they have the flu for a year. In combination with ribavirin (a nonspecific antiviral drug that isn’t effective on its own), peginterferon’s Sustained Virologic Response (SVR) rate was around 50% of those treated in clinical trials, and more than half of patients didn’t qualify for treatment due to serious side effects. Because of those same side effects, it’s hard to get patients to stay with treatment, which means effectiveness can be as low as 14% in urban, minority populations that are often struck hardest by the disease.
CTAF’s meta-analysis estimated the SVR of PR at 47% in clinical trials, leading to an NNT of 2.1 for each SVR. Pinning down costs can be a fool’s errand, but CTAF (via Red Book Online) estimated costs of approximately $1,000 per week for a 48-week course of treatment, or roughly $48,000 for a whole course. That means there’s a cost of about $102,000 per SVR on PR.
We’ll use those to start a table that we’ll fill in throughout the series. It’s important to note that the table is only for patients with genotype 1 infections who are eligible for PR therapy, and uses efficacy data drawn from CTAF’s meta-analysis. While this allows us to draw conclusions for best-case scenarios for the most common type of infection, things are different when not all these assumptions hold.
2011-2013: The DAAs arrive
In May 2011, the FDA approved the first so-called “direct acting antivirals” (DAAs): telaprevir and boceprevir. These drugs, called “direct acting” because they target the virus itself rather than the body’s response to it, were far more effective than PR alone, reaching SVR in roughly 75% of cases. But they were far from perfect: they still required patients to use the PR regimen in addition to the new drug, they had serious side effects, and they were still difficult to take, requiring regular dosing every 7-9 hours.
Plus, they weren’t cheap. CTAF estimated that telaprevir cost about $5,000 a week when it was the standard of care. Using contemporary guidelines, that meant a total cost of anywhere from $84,000 to $110,000 for the most difficult cases. That’s a huge increase from the PR regimen, but with a big jump in effectiveness too. For the purposes of updating our chart, this translates into an NNT of 1.3, with a cost per SVR of about $112,000.
So that (roughly) covers what treatment options looked like through 2013. In the next post, we’ll discuss sofosbuvir, which was approved by the FDA in December 2013.