The following is part of a series of guest posts on hepatitis C by Allan Joseph, a medical student at the Warren Alpert Medical School of Brown University and TIE research assistant. You can follow Allan on Twitter: @allanmjoseph. Links to all posts in the series to which this post belongs are in the introductory post. That post includes a glossary of terms as well.
If you listen to its supporters — and there are many, even outside of Gilead Sciences’ headquarters — sofosbuvir changed the hepatitis C (HCV) game for two reasons. First, in clinical trials, it had Sustained Virologic Response (SVR) rates of 90% — far and away the most efficacious HCV drug to that point, even in patients who had failed prior therapy. For comparison, the previous first-line treatments, telaprevir and boceprevir, had SVR rates of roughly 75%.
Second, sofosbuvir was much easier to give: it’s a once-daily pill, and for many patients with infections of genotypes 2 and 3, sofosbuvir did not require PR, making it much easier to tolerate, and opening treatment up to many patients who were previously ineligible. Those genotypes are generally considered more responsive to treatment, and therefore, researchers were able to test sofosbuvir as a standalone treatment. This also suggests sofosbuvir should have effectiveness much closer than to the efficacy in clinical trials; PR therapy had significantly lower effectiveness because its side effects made sticking to the treatment regimen difficult.
Here’s a problem, though: the major paper reporting landmark clinical-trial results for sofosbuvir actually reported the results from two separate trials. One trial, called NEUTRINO, was a single-group study of sofosbuvir in combination with PR in genotype 1, (and some others, 4 and 6, that we’re largely ignoring in this series for simplicity) — the more “difficult” infections. NEUTRINO reported the big headline number — a 90% SVR. The other trial, FISSION, compared sofosbuvir alone to PR therapy alone in genotypes 2 and 3 — the “easier to treat” infections. It was a randomized “noninferiority” trial and reported that sofosbuvir alone was, in fact, equivalent to PR alone in genotypes 2 and 3. That is, patients with GT2 or GT3 infections could use sofosbuvir to avoid PR side effects (improving adherence) with the same expectation of a cure.
Notice the problem? Those aren’t comparable results because they studied different regimens in different populations, but the two distinct benefits of sofosbuvir (higher efficacy and lower side effects) are often conflated. It’s obvious that sofosbuvir can improve therapy in hepatitis C, but depending on to whom it’s applied, it improves it in different ways.
For patients with GT1 infections, the evidence suggests adding sofosbuvir to PR will improve treatment and SVR rates. For patients with GT2 or GT3 infections, sofosbuvir alone is equivalent to the PR regimen — and according to the very latest paper on this topic, adding ribavirin to sofosbuvir for these patients can boost SVR rates into the 90-percent range while avoiding the side effects of peginterferon.
Regardless, it’s the costs, not the efficacy data, that are making a big splash in the popular media. Sofosbuvir is a $7,000-a-week treatment that can last anywhere from 12 to 24 weeks, and some regimens still use PR. Based on current guidelines, though, a course of sofosbuvir treatment can run anywhere from $84,000 to nearly $180,000 in the most expensive patients. Those are the patients who were previously ineligible for PR-based treatment and have particularly difficult to treat forms of the infection — they still can’t take PR, but their courses are twice as long as those of other patients, which might still involve the relatively cheaper PR.
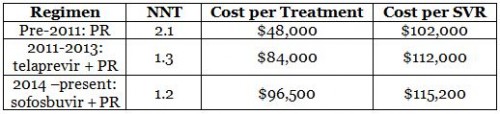
To continue the chart we began developing last post, the NNT for sofosbuvir in the same population (genotype-1, PR-eligible patients) is 1.2, leading to a cost per SVR of $115,200.
As you can see from the chart, the California Technology Assessment (CTAF) analysis suggests that the cost per SVR is actually about the same as telaprevir’s. Moreover, in real-world effectiveness, sofosbuvir is more likely to be taken regularly than telaprevir because of its once-daily dosing, meaning sofosbuvir’s cost-per-SVR in the real world could be much closer to what clinical trials suggest than other regimens.
One other thing to consider is that sofosbuvir might, in fact, be cost-saving: that is, it’s possible the drug is so effective that it pays for itself in the form of reduced spending later in life. The CTAF analysis suggests that the net present value of savings over a 20-year horizon is about 75% of the cost of treating all hepatitis C patients with sofosbuvir. Various other studies suggest that under certain reasonable assumptions (though by no means the only reasonable assumptions), sofosbuvir may pay for itself over the long run. That’s a pretty remarkable finding.
If sofosbuvir’s financial savings are actually close to its costs, then it’s quite likely that it is at least cost-effective. We’re asking if all of the non-pecuniary benefits — avoiding PR therapy, shorter courses of therapy, improved quality and length of life — are worth whatever extra costs sofosbuvir may bring. Given how rough PR treatment is, and sofosbuvir’s ability to treat PR-ineligible patients, it’s reasonable to argue that approving sofosbuvir is cost-effective. Three different papers have roughly estimated that the total value of sofosbuvir’s benefits exceeds its costs. Whether states and insurance companies will agree, however, is a different story, and it’s one I’ll discuss later in this series.
For now, there’s some debate over whether the evidence for sofosbuvir’s efficacy is as strong as suggested. If the efficacy numbers above aren’t to be trusted, then there’s a bigger problem in play. We’ll go into that in the next post.