The current NEJM has published a clinical trial with a statistical commentary that is really exciting. (How is that for a sentence that you never expected to read?) Bear with me while I present the background problem and explain why the trial may mark a breakthrough in how quickly we can change medicine.
The interminable battle over the ACA suggests that the only significant problem in health care is how we finance it. That’s wrong: the real problems are that the care isn’t good enough, and yet it costs too much. The most important issues in health care are improving the patient experience of care, reducing the per capita cost of care, and — above all — improving the health of populations. You can’t achieve these goals through legislation.
These goals can only be achieved by learning how to deliver more effective care at lower cost. How does such learning happen? Partly through advances in basic science that lead to better treatments. Advances in basic science, however, do not translate directly into effective treatments. You have do a lot of clinical research to develop a new therapy and eventually you have to test that therapy on patients in clinical trials. The rate at which you can run clinical trials is therefore a rate limiting step on how quickly the health care system can learn and improve.
It’s valuable to think about the health care system as an adaptive learning system. We can then look for ways to reorganize the system so that it can learn more quickly. At the end of the day, the clinical trials process is more or less trial and error learning. And the only way to speed up trial and error learning is to run a lot of trials and run each trial more quickly.
Here we reach a deep problem: clinical trials are slow and expensive.* Trials take years to organize and once started they take years to recruit enough patients to adequately test the new treatment. Each patient costs tens of thousands of dollars to run, much of it in the cost of finding and recruiting the patient, and the cost of the data collection to assess the patient and the outcomes of her treatment.
There are other problems with clinical trials. Trials are frequently designed to recruit groups of nearly homogenous patients. Using groups of highly similar patients makes the data cleaner. Clean data maximizes the statistical power of the trial, which helps keep the cost down. But the consequence is that the trial is run on a subpopulation of patients that is unlike the more heterogeneous patients who are actually seen in clinics. That means it is hard for a doctor to know whether the result of the clinical trial actually applies (‘generalizes’) to the patient she is treating.
And now we get to the study in the NEJM. The TASTE study (Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction) was testing a tweak (thrombus aspiration) in the technique for percutaneous coronary intervention (PCI), a common treatment for heart attack patients. The news about thrombus aspiration is that it doesn’t work. Unless you are an interventional cardiologist, that’s not interesting. What’s interesting is how they did the trial.
The trial was conducted in Sweden, where there is a national registry of angiography and angioplasty patients. That is, all the patients who received these cardiological treatments are enrolled in a database. Records about them are kept in a standard way across the country. There is routine follow up with standard measures so that Swedish doctors know what eventually happens to all these patients. This is incredibly valuable for studies of the cost and quality of care, for assessing drug safety, and for retrospective analyses of the outcomes of care. The Swedes have registries for all kinds of procedures and it helps them achieve high quality of care.
What the TASTE study did was to run the trial using the national registry data. That is, they recruited patients them from the list of patients in the registry. Patients were randomized to either standard PCI or PCI with thrombus aspiration. The trialists then used the registry data to describe the clinical and personal histories of the patients. They used the outcomes data collected by the registry as the trial endpoints. Using the registry meant that the trialists could find patients faster because they just had to look them up. They could run each patient much more cheaply because most of the critical data were already being collected. And they knew exactly how representative the clinical sample was because the entire patient population is in the registry. So the study was done quickly and cheaply, and the results are generalizable.
There is, of course, an up front cost to set up the national registry. Before anyone complains about this, be aware that care is not only better in Sweden, it’s a lot cheaper.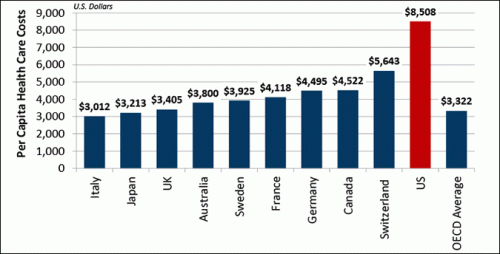 The costs of establishing and running a registry are mostly getting everyone to keep records in a standard way and regularly following up patients to measure clinical endpoints. The thing is, we should be doing these things anyway.
The costs of establishing and running a registry are mostly getting everyone to keep records in a standard way and regularly following up patients to measure clinical endpoints. The thing is, we should be doing these things anyway.
The lesson from the TASTE study is that we should implement registries throughout the US and Canadian health care systems and use them to run quick and efficient clinical trials. That will help us adapt our way to a health care system that works well at an affordable cost.
* See Avik Roy here for a criticism of the FDA’s role in slowing the pace of clinical trials research.

