Personalized medicine is expected to revolutionize health care by developing new molecular tools to precisely diagnose and treat the problems of individual patients. But how will these new technologies affect the cost of care? Changing the cost of care will in my view require more than just changing how medical care is paid for: We also have to develop more efficient treatments and delivery systems. The story of a new treatment for cystic fibrosis (CF) may give us some idea about what will be possible in the near term.
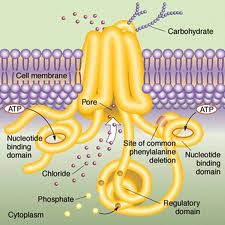
CF is a rare (1 in 4,000 US children) genetic disorder of the gene for the protein ‘cystic fibrosis transmembrane conductance regulator’ (CFTR). Defects in that protein cause abnormal transport of chloride and sodium across an epithelium. This in turn causes dysfunction in the lungs, pancreas, liver, and intestine. In particular, it results in the development of thick mucous secretions in the lung. These secretions become a site for infection. The infections cause diseases such as pneumonia, leading to reduced life expectancy.
Previously, caregivers could only address the symptoms of CF, for example by keeping the patient’s lungs clear, keeping the patient fit, and by aggressively treating the infections. They could not address the disease at the cellular level. However, clinicians have been extremely successful at managing symptoms and life expectancy at birth for US patients has increased from just months in the 1950s to over 50 years today. Much of this has been achieved through close cooperation between CF caregivers and CF families.

There is also great interest in attacking the disease at a molecular level. The CFTR gene was discovered in 1989. Since then, there have many efforts at developing either a gene therapy (without success) or a medication that can address the epithelial transport problems at the origin of the disease’s causal chain. Among many challenges is that there are more than 1500 mutant alleles, so in effect, the already small ‘CF population’ is actually a cluster of many tiny genetic subpopulations.
One research group picked the mutation G551D-CFTR, which occurs in 4-5% of CF patients. With funding from the CF Foundation, they tried over 600,000 compounds and eventually developed a drug named Kalydeco (ivacaftor). Results of a clinical trial were published in the New England Journal in 2010 and FDA approval was obtained in January of 2013. So far this effort has been a success, producing a significant improvement in lung function for patients. So, just as the CFTR was one of the earliest disease genes to be mapped, Kalydeco is one of the first successful medications produced by genomic medicine.
That is the good news. This bad news is that it took 24 years and tens of millions of dollars to get from the discovery of the CFTR to the FDA approval of a drug. Moreover, this drug was designed for a mutation found in only a small fraction of the population of an already rare disease. Most importantly, the drug reportedly costs $300,000 / year, in part because the market is likely small. (These costs may be offset in part if the patients taking it have fewer hospitalizations or are less likely to need a lung transplant.)
Future drug development in personalized medicine might get cheaper. Still, precisely because the treatments are targeted at phenomena at the level of specific harmful mutations, they are not just personalized but practically bespoke, and correspondingly pricey. The CF story therefore gives us reason to fear that personalized medicine will make health care more rather than less expensive
So what should we do? I am hopeful about personalized and genomic medicine: I am part of a group that has been working on the development of gene expression biomarkers for depression. More importantly, even if genomic medicines prove to be very expensive that may be okay if they are also very effective treatments. What matters is not the cost but whether we get real value for the money.
Having said that, if we are going to bend the cost curve down, we will probably need to do it in the face of upward health cost pressure from personalized medicine. But I think the big lesson from cystic fibrosis is how much can be accomplished through low-tech but intensive management of a chronic disease. We need to find better ways for patients to take care of themselves and better ways for clinicians to help them do this. Finding better ways to build partnerships between clinicians and families does not have the scientific glamour of molecular medicine, it will not garner Nobel prizes, and it will not create profitable intellectual property. But it can be extraordinarily effective at saving lives.
@Bill_Gardner

