The controversial FDA Avastin hearings were yesterday and today. (Prior TIE coverage here) My expectations for evidence-based reporting were low. But with the exception of the Wall Street Journal opinion page, the print media did a reasonable job. A limited sample follows.
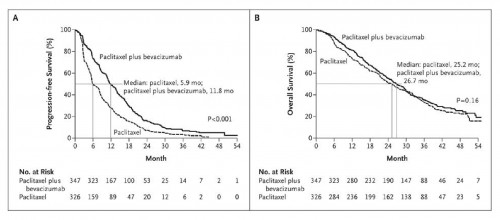
Just as a refresher, here are the original charts published in the NEJM in December 2007 that were used to justify the provisional Avastin approval for metastatic breast cancer. The data shows statistically significant “progression-free survival” but no improvement in survival. The follow up studies failed to replicate Chart A:
Anna Yukhananov at Reuters wins first prize for a clear, unbiased narrative of the hearings Tuesday and Wednesday. The main point of the story is summarized in the first bullet point at the top:
* Patients say Avastin helps, data shows little benefit
I also appreciate the subtle but important point that the FDA decision won’t necessarily foreclose off-label use and reimbursement. She quotes an investment analyst saying that even if the FDA modifies the label, there is a “60% chance” that private insurers will still cover it. Given the science, one wonders why they would:
Avastin won U.S. clearance for breast cancer in 2008 based on a study showing the drug stalled cancer growth by 5.5 months more when used in combination with standard chemotherapy. As part of an accelerated approval, the FDA required Roche to run follow-up studies to confirm the drug worked.
Later studies found only a one- to three-month delay in breast cancer growth. None of the studies showed Avastin extended the lives of patients with advanced breast cancer.
Over at the Wall Street Journal, we see science on trial in an opinion piece. The article is headlined “The Race Against The Cure” which presumes that Avastin is indeed a cure. The FDA is styled as “de facto prosecutors” and “bureaucrats” “known for punishing those who challenge it.” The FDA process is derisively called a “ukase” which was an imperial Russian diktat from the Tsar. Then the attack gets really personal:
FDA cancer drugs chief Richard Pazdur has enormous discretion in drug approvals, and he can be especially vindictive… Dr. Pazdur rigged the review process against Avastin.
Elsewhere at the Wall Street Journal, health reporter Alicia Mundy does a much better job describing the hearing, but she is a bit slow and thin when describing the science. Her story opened as follows:
Breast-cancer patients who say Roche Holding AG’s Avastin saved their lives opened an appeals hearing on the drug Tuesday, calling on the Food and Drug Administration to let the “miracle” medicine keep its approval.
The FDA, which has emphasized concerns about whether the drug works on breast cancer, took off the gloves Tuesday to talk about the drug’s risks. An agency medical official described women who suffered fatal hemorrhages or other side effects from the drug and argued that it didn’t deserve approval in breast cancer.
The hearing is drawing close attention from cancer patients and the drug industry because it is a rare challenge to the FDA’s authority to make up-or-down decisions on drugs for life-threatening diseases, particularly cancer. Critics say the agency is too tough on new treatments, while backers contend the FDA is standing up for science against a political onslaught.
The key to the FDA’s case isn’t side effects in terminal patients, but lack of clinical effectiveness and therefore side effects despite no improvement in longevity. You need to read down to paragraph six to hear about the studies, and the review of the clinical trials is incomplete:
The drug is approved for four other forms of cancer and will stay on the market regardless. FDA officials say that in breast cancer, studies show the drug delays tumor growth only for a month or so in most women and doesn’t extend lifespan.
The National Journal highlighted the personal struggles for breast cancer patients and sensational attacks by Avastin supporters, but balanced the story with facts:
Dr. Milton Wolf, a radiologist who writes columns for the conservative Washington Times newspaper, called the FDA committee a “death panel” last week.
Of course, it’s the FDA’s job to do precisely what it is doing–decide which drugs are safe and effective enough to use and which are not. FDA Commissioner Margaret Hamburg will make the decision on Avastin after reviewing the evidence presented at the meeting on Tuesday and Wednesday.
Strong evidence shows that while Avastin can have remarkable effects on some tumors of the colon, brain, and elsewhere, it doesn’t help breast cancer patients live any longer, and it can in fact kill some people. It is the world’s best-selling cancer drug, but it also has side effects ranging from internal bleeding to high blood pressure.
“Patients with late-stage cancer are often desperate for help and grasp at straws,” Richard Deyo of the Oregon Health and Science University told The Washington Post last year. “Without this regulatory move, the drug would continue to be used, and would presumably not benefit anyone and add a lot of treatment costs.”
… Some oncologists say that there may be some “super-responders” who benefit from the drug, but the FDA’s Patricia Keegan said at the meeting on Tuesday that she saw no evidence of this in any of the studies.
Over at CNN, you have to read down to paragraph six before being told the key data on the clinical trials:
“Four clinical studies showed that the drug did not, on average, make patients live longer.”
The balance of the long article covers the issue thoroughly.
Andrew Pollack at the NYT opened with these three paragraphs:
With desperate breast cancer patients imploring the Food and Drug Administration to change its mind, the agency’s staff calmly argued Tuesday that the drug Avastin should lose its approval as a treatment for that disease.
The pleas and presentations came on the first day of a two-day hearing at which Genentech, the manufacturer of Avastin, is getting a chance to try to persuade the F.D.A. to reverse its decision made in December to revoke the drug’s approval for advanced breast cancer.
The proceedings emphasized a conflict that has bedeviled pharmaceutical regulation and other efforts to control the practice of medicine — one between cold statistics from clinical trials about overall populations and the often emotional experiences of individual patients who say a therapy has, or might, work for them.
Pollack clearly describes the clinical trials later in the article:
Avastin received so-called accelerated approval for metastatic breast cancer in 2008 under a system intended to allow drugs for serious diseases to get to market more rapidly, subject to later studies to confirm they really work.
The F.D.A. said that those subsequent studies had not confirmed that Avastin was safe and effective. With five randomized trials of Avastin now having been completed, the F.D.A. said, no trial had shown that Avastin prolonged life or improved the quality of life. And no trial showed that the drug delayed the progression of tumors to the same extent as the one trial that led to the drug’s approval.
“All we are asking for here is one trial that shows clinical benefit,” said Dr. Richard Pazdur, the head of the agency’s cancer drug division. (emphasis mine)
Pollack also describes reimbursement and off-label use in clear laymen’s terms:
Even if the approval is revoked, Avastin would remain on the market as a treatment for other types of cancer, so doctors could use it off-label to treat breast cancer. However, insurers would be less likely to pay for the drug, which Genentech says costs a typical breast cancer patient $88,000 a year.
As for blogs, GoozNews has a good science-based approach to Avastin. Merrill is always interesting to read.
UPDATE 1: from the comments, more blogs on the Avastin hearing – The Dismal Political Economist and FAMEDS, a very active patient group supporting Avastin for metastatic breast cancer. I’m not planning on a score card for the blogs.
UPDATE 2: the vote is 6-0 against Avastin.
UPDATE 3: from the comments, a recent perspective on this Avastin debate in the NEJM
UPDATE 4: today’s SF Chronicle story slants towards home-town favorite Genentech. The first paragraph said:
A panel of cancer experts ruled Wednesday that Avastin – the world’s best-selling cancer drug, developed by Genentech in South San Francisco – should no longer be used in breast cancer patients because of concerns the medicine didn’t work as well in follow-up studies and may cause deadly bleeding.
But the data is stronger than “concerns the medicine didn’t work as well in follow-up studies” – the follow up studies failed to confirm that the drug worked at all in metastatic breast cancer. In the second and third paragraphs, the story correctly described the next procedural steps, the option for off-label use, and the possibility of reduced reimbursement. The third paragraph emphasized safety, again without discussing the alarming efficacy data:
The decision capped two days of testimony from patients urging the panel to continue access while FDA scientists argued the drug is too dangerous.
You have to wait until the sixth paragraph to hear about the efficacy problem:
The advisers also voted unanimously that Avastin didn’t have benefits in breast cancer and that its risks weren’t justified.
Reporters, please tell your readers what the scientific evidence says.