That’s the title of a new piece at the New England Journal of Medicine’s Catalyst, and it’s from Amitabh Chandra, Craig Garthwaite, Ariel Dora Stern, and me.
While the Orphan Drug Act has stayed the same, drug markets have changed dramatically over the past 3 ½ decades. Most significantly, the market price for orphan drugs is now remarkably high: in 2017, the average price for the top 100 orphan drugs was $147,308 a year per patient. (This is only an estimate.) Manufacturers have been accused of slicing prevalent diseases into smaller subcategories, sometimes characterized by genomic biomarkers, allowing firms to secure ODA incentives for a drug that is likely to be used in a large patient population. And the growing use of auxiliary or “surrogate” endpoints in clinical trials for orphan drugs has reduced the time and expense required for clinical research, because they set lower thresholds for indicating treatment success—e.g., slowing a tumor’s growth vs. longer survival.
As a result of these trends, the Orphan Drug Act rewards some drug manufacturers for bringing drugs to market that in all likelihood would have been produced without additional incentives. The incentive structure is an especially poor fit for orphan drugs that target such rare conditions, or are so challenging to manufacture, that the market will not support similar products from multiple firms. Because these “natural monopoly” drugs will never face meaningful competition, they may be very lucrative even without the ODA benefits.
With some regularity, manufacturers will also secure FDA approval for new orphan indications for a drug that’s already in widespread use. That practice has been tarred as abusive: if we already know the drug works for that particular indication, we don’t learn much from studies that drugmakers conduct to demonstrate the drug’s efficacy for that indication. We’re rewarding wasted effort.
That’s often true. But it’s not always true. Take adalimumab (Humira), for example. It’s a blockbuster drug with multiple orphan indications. For most indications, we found that orphan drug approval didn’t yield any change in prescribing patterns. That suggests clinicians didn’t learn anything new from the orphan studies. But after approval for one indication—a skin disorder called hidradenitis suppurativa—usage increased. In that case, if not the others, the Orphan Drug Act’s incentives appear to have spurred some useful clinical research.
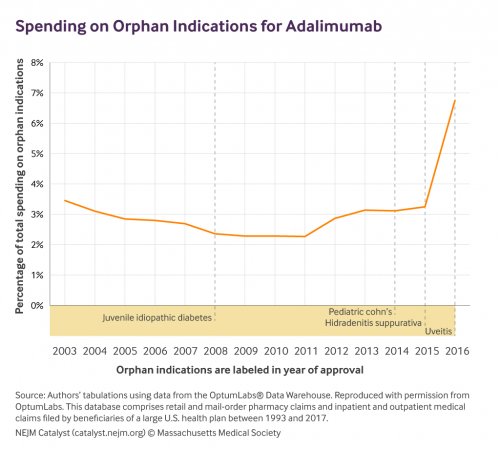
There’s a lot in the paper, which I suspect will be somewhat controversial. But I think we make sound suggestions about how to reform an Act that’s getting a bit long in the tooth.


