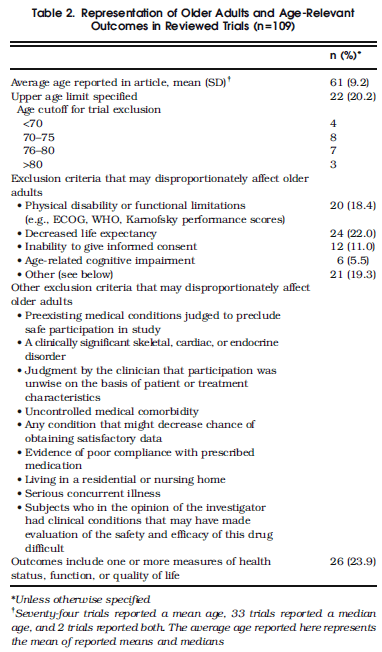
A study from Zulman et al.* in the latest Journal of General Internal Medicine reports on the incidence of excluding the elderly from clinical trials. The study reviewed papers published in 2007 in several top** journals (N=109 studies met screening criteria for inclusion). Around one fifth of the studies excluded patients above a certain age, while nearly half had exclusionary criteria that disproportionately excluded older adults. Table 2 from the study below describes the exclusionary criteria commonly used.
 Exclusions from studies are designed to protect patients from unwarranted harm, but may also be used to make a study easier/safer for research staff to complete. Both of these reasons are legitimate, but it is worrying for studies to exclude persons in age ranges and with conditions who will be using the therapy being tested. A more negative interpretation for exclusions is that it could represent cherry picking of patients to improve the odds of obtaining a result that is beneficial to a pharmaceutical or therapy being assessed. It is hard to distinguish between these motivations.
Exclusions from studies are designed to protect patients from unwarranted harm, but may also be used to make a study easier/safer for research staff to complete. Both of these reasons are legitimate, but it is worrying for studies to exclude persons in age ranges and with conditions who will be using the therapy being tested. A more negative interpretation for exclusions is that it could represent cherry picking of patients to improve the odds of obtaining a result that is beneficial to a pharmaceutical or therapy being assessed. It is hard to distinguish between these motivations.
Excluding patients in older age ranges or with chronic conditions disproportionately effects the Medicare population. The average of the mean or median age of the 109 studies assessed was 61 (sd 9.2) [really an average of the average]. The exclusion criteria “inability to provide informed consent” makes little sense to me as a special exclusion criteria, because I understand that informed consent must always be given for a patient to be enrolled in a research study. However, the other exclusionary criteria remove patients from studies, but patients with those conditions will be treated with the therapy being tested. This means that clinical decisions are often being made without the best evidence and/or using old evidence. For example, excluding patients with physical disability or functional limitations is going to disproportionately exclude not only Medicare beneficiaries, but dual eligible beneficiaries who are also covered by Medicaid due to long term care needs in many cases. Such patients are the sickest, most expensive patients in the system on a per capita basis.
Randomized Control Trials are the gold standard for determining internal validity–does x that is randomly assigned, cause y? However, the external validity of trials is also important–how applicable are the findings of a particular study for the population who needs the therapy? I think reduced external validity due to excluding certain types of patients is an under-appreciated problem in research.
Recently I have been involved in the design of two clinical trials where I was providing health policy/economics expertise for secondary analysis. In both cases, there were discussions of exclusionary criteria that didn’t make sense to me because I thought they limited the usefulness of the trials in question from an external validity perspective. In one example, we were designing a randomized trial of early palliative care for persons with Congestive Heart Failure (CHF). This is an attempt to replicate the trial of early palliative care in stage IV lung cancer by Temel et al. reported around a year ago. My biggest worry was the exclusion of patients who had been approved for heart transplant and/or the use of left ventrical assistive device (LVAD) as a destination therapy, meaning using the therapy not as a bridge to a transplant.
In my thinking, these are the exact patients who needed to be randomized to a work up by the palliative care service to make sure that they are having their physical symptoms as well as the complex psycho-social needs of both patient and family being addressed. Further, a clear assessment of goals of care seems warranted for patients about to undergo such an intensive therapy to make sure that the patient understands to what they have consented. If some change their mind after a palliative care work up then that is their choice, and part of the point.
In the other instance, we were designing a statin discontinuation trial that had exclusionary criteria that seemed to render the trial to essentially be a statin discontinuation trial for persons with advanced cancer. I don’t think it would be a problem to conduct a trial that was basically trying to see the impact of stopping this common therapy among persons with a very short expected lifespan due to cancer. But, the trial wasn’t focused on that explicitly, and in theory anyone with very short lifespan was eligible though I worried whether that was true in reality.
In both cases, the smartypants health policy/economics member of the team (me) didn’t win the day on getting rid of the exclusions. And I am not a clinician, so won’t win those sorts of fights, and shouldn’t. However, it seems to be a problem if we exclude patients from studies if people suffering from the same excluding diseases will be treated by the drugs or therapies being tested in the studies. We have to keep these issues in mind. It is first and foremost about truncating the evidence base that we have.
*Donna M. Zulman, Jeremy B. Sussman, Xisui Chen, Christine T. Cigolle, Caroline S. Blaum, and Rodney A. Hayward. Examining the Evidence: A Systematic Review of the Inclusion and Analysis of Older Adults in Randomized Controlled Trials. 2011; J Gen Intern Med (26)7:783–90
**Journals included: JAMA, NEJM, Lancet, BMJ, Circulation.

